II. Manageable Components of the Composting Process
|
| While the natural process of decomposition will
occur without any assistance from us, several factors can be
managed to accelerate the compost process. (Substitution -
original slide not available in electronic form). |
 |
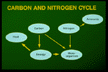
| Organisms utilize carbon as a source of energy
and nitrogen to grow and reproduce. Without enough nitrogen,
there will be few microorganisms, and decomposition will be slow.
If there is too much nitrogen in the compost, some of it will
turn to ammonia that will volatilize, creating an odor. |
 |
| The optimum C:N ratio is about 30 to 1. This
ratio will make fast, hot compost. Grass, animal manures and
fresh green plants are high in nitrogen. |
 |
| Leaves, brush, sawdust and wood chips are all
good sources of carbon. Blending these carbon sources with nitrogenous
materials can provide a satisfactory C:N ratio. |
 |
| Surface area is another key factor to consider. Since decomposition
is a microbiological process, it occurs in thin films on the surface of
particles. A large particle has less total surface area than the same particle
chopped into small pieces. Therefore if particles are too big, the process
will take longer. A one-inch wood chip will decompose much slower than grains
of sawdust. An easy way to shred fallen leaves is to mow them before raking. |
 |
| Decomposer organisms need water also. The decomposition
process will slow down with either too much or too little water.
The optimum moisture content for compost is about 40 to 60 percent,
damp enough so that a handful feels moist to the touch, but dry
enough that a hard squeeze produces no more than a drop or two
of water. |
 |
| Most microorganisms active in composting require
oxytgen to live. Their "aerobic" activity forms carbon
dioxide and heat as by-products. If too little oxygen gets into
the compost, the process can become "anaerobic." This
condition results in foul odors. The by-products of anaerobic
decomposition include methane and hydrogen sulfide gas. Hydrogen
sulfide smells like rotten eggs. |
 |
| Oxygen will move into the pile if it is loose and there is
plenty of space between particles, as when straw is mixed in the pile. Finer
material may need to be aerated by physically turning the pile with a pitch
fork or a compost turning tool. With the rapid decomposition that occurs
with high nitrogen materials, turning the pile becomes necessary to prevent
anaerobic conditions from developing. |
 |
| Heat will be given off as organisms feed on wastes
and break them down into less complex molecules. Ideal temperatures
for composting are between 90 and 150 degrees Fahrenheit. High
temperatures can help kill weed seeds and disease organisms,
but temperatures above 150 degrees Fahrenheit will also kill
the decomposers and slow the process. |
 |
| Compost piles should be a minimum of one cubic yard in size.
Smaller piles may not have enough mass to hold the heat of decomposition. |
 |
| Bacteria reproduce very quickly and are naturally
present in air and soil, so there is usually no need to add them
to the compost pile. Of the many inoculants, or compost starters
available, the best is a handful of freshly made compost. |
 |











