
Ammonia is among the most common odors found at composting facilities. Fortunately, ammonia is not a pervasive odor, so it does not require a large number of dilutions to reduce concentrations below the odor threshold. Ammonia also disperses easily, since is lighter than air (its density is 60% that of air), and does not settle in low lying areas the way hydrogen sulfide and other dense odorous compounds do. These factors make ammonia odors more prevalent on-site than off-site.
Ammonia odors can be formed aerobically as well as anaerobically, so the control strategies recommended for anaerobic odors may not apply. Noticeable ammonia losses primarily result from a low C/N ratio. The microorganisms are very efficient at utilizing nitrogen when that is the limiting nutrient. The smell of ammonia is an indicator that nitrogen is in excess, and carbon/energy is limiting instead. Ammonia losses are common when composting high nitrogen materials such as fresh grass clippings or manure, and are often accompanied by other nitrogen losses in runoff or infiltration. At large composting facilities these nitrogen losses could threaten surface or groundwater quality.
Another factor affecting the magnitude of ammonia volatilization is pH. NH3 (gaseous ammonia) and NH4+ (aqueous ammonium ion) are in equilibrium at a pH of about 9, with higher pH's forcing more NH4+ into the gas form that you can smell. Thus ammonia is rarely noticed if the pH is acidic , and adding lime to a pile will increase the ammonia odor. The equilibrium relationship is defined by the following equation.
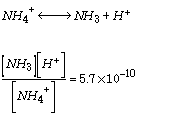
Source: Sawyer and McCarty, 1978)
A plot of this equation, showing the relative concentrations of NH3 and NH4+, is provided below.
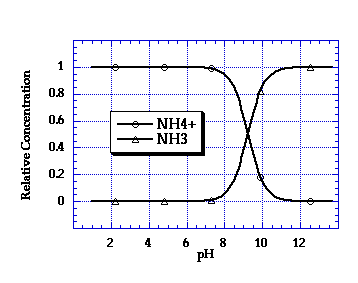
In a real composting system this equilibrium relationship would have to be corrected somewhat if there are other ions in solution, or if the compost is at a temperature other than 25°C.
Zeolites (natural ion exchange resins/minerals) can be used to trap excess nitrogen, and are being tried on a pilot basis at a few composting facilities. In this case it is the ammonium ion form that is bound. Assuming pH doesn't change, lowering the NH4+ concentration will also reduce the NH3 concentration proportionately, as the two forms adjust to a new equilibrium. The zeolite that is most effective at trapping ammonium ions in wastewater applications is clinoptilolite. Composters should be sure to avoid the common water softener Sodium Zeolite, as the sodium will be released into the compost rendering it too salty for use (Burkhardt, 1995). Interestingly, in wastewater applications the zeolite is usually regenerated using lime - Ca(OH)2 - in which calcium (Ca++) replaces the ammonium ions (NH4+) and converts them to gaseous ammonia (NH3), which is then discharged to the atmosphere. Obviously we don't want this regeneration process to occur in a composting pile, so be careful of this approach with high lime materials.
Acknowledgments
Helpful reviews and discussions related to this document were provided by James Gossett, Nancy Trautmann, Daniel Cogan, and Cary Oshins.
References
Peter Burkhardt, e-mail communication, December 1995
Sawyer, C.N and P.L. McCarty, 1978. Chemistry for Environmental Engineering, 3rd Edition. McGraw-Hill Book Company, NY, NY. 532 pp. Return to citation in text.
|
Composting |
Engineering |
in Schools |
|
For specific comments related to this page, please contact the Cornell
Waste Management Institute (format and style), or Tom
Richard (technical content).
Cornell Waste Management Institute © 1996
Cornell University
Ithaca, NY 14853
607-255-1187
cwmi@cornell.edu